- Research
- Open access
- Published:
Cost-effectiveness of adrenaline for out-of-hospital cardiac arrest
Critical Care volume 24, Article number: 579 (2020)
Abstract
Background
The ‘Prehospital Assessment of the Role of Adrenaline: Measuring the Effectiveness of Drug Administration In Cardiac Arrest’ (PARAMEDIC2) trial showed that adrenaline improves overall survival, but not neurological outcomes. We sought to determine the within-trial and lifetime health and social care costs and benefits associated with adrenaline, including secondary benefits from organ donation.
Methods
We estimated the costs, benefits (quality-adjusted life years (QALYs)) and incremental cost-effectiveness ratios (ICERs) associated with adrenaline during the 6-month trial follow-up. Model-based analyses explored how results altered when the time horizon was extended beyond 6 months and the scope extended to include recipients of donated organs.
Results
The within-trial (6 months) and lifetime horizon economic evaluations focussed on the trial population produced ICERs of £1,693,003 (€1,946,953) and £81,070 (€93,231) per QALY gained in 2017 prices, respectively, reflecting significantly higher mean costs and only marginally higher mean QALYs in the adrenaline group. The probability that adrenaline is cost-effective was less than 1% across a range of cost-effectiveness thresholds. Combined direct economic effects over the lifetimes of survivors and indirect economic effects in organ recipients produced an ICER of £16,086 (€18,499) per QALY gained for adrenaline with the probability that adrenaline is cost-effective increasing to 90% at a £30,000 (€34,500) per QALY cost-effectiveness threshold.
Conclusions
Adrenaline was not cost-effective when only directly related costs and consequences are considered. However, incorporating the indirect economic effects associated with transplanted organs substantially alters cost-effectiveness, suggesting decision-makers should consider the complexity of direct and indirect economic impacts of adrenaline.
Trial registration
ISRCTN73485024. Registered on 13 March 2014.
Introduction
Although survival rates following adult out-of-hospital cardiac arrest have increased in recent years, they generally remain below 10% [1, 2]. Survivors are at increased risk of cognitive and functional impairment and often take significant time to attain an acceptable quality of life or to return to work [3].
In some countries, when a patient dies after cardiac arrest, families can be asked to agree to the patient’s organs being donated for the benefit of others. A meta-analysis of 26 studies reported that, on average, 5.8% (95% CI 2.1–10.9%) of patients admitted to intensive care donate their organs for transplantation after brain stem death [4]. Observational studies suggest that cardiac arrest before organ donation does not adversely affect long-term graft function [5] and that such transplant programmes are cost-effective [6, 7].
The PARAMEDIC2 (Prehospital Assessment of the Role of Adrenaline: Measuring the Effectiveness of Drug Administration In Cardiac Arrest) trial showed that adrenaline improves overall survival, but not long term neurological outcome [8, 9]. In this paper, we explore the costs and benefits of adrenaline within the PARAMEDIC2 trial across within-trial and lifetime horizons and model the contribution of organ donation after a cardiac arrest on cost-effectiveness.
Methods
Trial background
PARAMEDIC2 (ISRCTN73485024) was a pragmatic, individually randomised, double-blind, controlled trial of 8014 adult patients with out-of-hospital cardiac arrest in the UK [9]. Patients, treated by five National Health Service (NHS) ambulance services between December 2014 and October 2017, were randomised to either parenteral adrenaline or saline placebo, along with standard care. The primary clinical outcome was the rate of survival at 30 days. Survival, neurological outcomes as indicated by the modified Rankin Scale (mRS) (ranging from 0 [no symptoms] to 6 [death]) [10] and economic outcomes were followed up from randomisation to 6 months. The South Central–Oxford C Research Ethics Committee (REC: 14/SC/0157) and the Medicines and Healthcare Products Regulatory Authority (EudraCT: 2014-000792-11) approved the study protocol. The trial was funded by the UK National Institute for Health Research Health Technology Assessment Programme (12/127/126). Further details of the trial are reported elsewhere [9].
Overview of economic evaluation
A cost-utility analysis was conducted, with results expressed in terms of incremental cost per quality-adjusted life year (QALY) gained through the use of adrenaline. The baseline economic evaluation was conducted from the perspective of the UK NHS and personal social services (PSS) [11], with a time horizon of 6 months post-randomisation. A decision-analytic model was used to extrapolate outcomes beyond trial follow-up and assess the cost-effectiveness of adrenaline over a lifetime horizon. All costs were expressed in British pounds sterling and valued at 2017 prices (with commensurate values in Euros estimated using purchasing power parities). Costs and QALYs accrued beyond the first year of follow-up were discounted to present values at 3.5% per annum in accordance with recommendations from the National Institute for Health and Care Excellence (NICE) [11]. The economic evaluation adhered to an approved pre-specified analytical plan which is available in the supplementary material (Additional file 1).
Measurement and valuation of resource use
Resource inputs associated with the pre-hospital emergency response, until the point of hospital admission or death, were extracted from the trial case report forms completed by research paramedics. These data included the number of emergency response staff/ambulance crew and vehicles in attendance, duration of emergency response, and cumulative doses of adrenaline administered. Unit costs for these resource inputs were drawn from national tariffs (Additional file 2).
For patients surviving to hospital admission, the economic costs associated with hospital care were extracted through data linkage with two national population-wide databases: Hospital Episode Statistics (HES) for England and the Patient Episode Database for Wales (PEDW). This provided patient-level profiles of resource use associated with hospital episodes for study patients over the trial time horizon, including emergency department attendances, critical care and other inpatient admissions, covering procedures undertaken including percutaneous coronary intervention, by-pass surgery, implantable cardioverter defibrillators (ICDs), cardiac pacemakers and lengths of stay, day case admissions and outpatient attendances. HealthCare Resource Group (HRG) codes were derived using the NHS HRG4 2016–17 Reference Cost Grouper software version [12]. The Department of Health and Social Care’s Reference Costs 2016–17 schedule was used to assign costs to derived HRG codes [12]. For patients without linkage to routine hospital records, hospital resource inputs were extracted from data contained within the trial case report forms and valued using national tariffs.
Surviving patients or their proxies were asked to complete questionnaires at 3 and 6 months post-randomisation, reporting hospital admissions and use of hospital outpatient services following initial discharge, by type and volume, and use of community health and social services, medications and aids and equipment. Further questions captured wider societal attributable resource use, with data collected on time off work, over-the-counter medications, aids and adaptations purchased privately and any additional costs borne by study patients or informal carers. Unit costs for these resource inputs were drawn from national tariffs (Additional file 2).
Calculation of health utilities and QALYs
Health-related quality of life was assessed using the EuroQol EQ-5D-5L [13], completed at 3 and 6 months post-cardiac arrest. Responses to the EQ-5D-5L descriptive system were converted into health utility values, based on the UK EQ-5D-3L tariff using the van Hout interim cross-walk algorithm [14], in line with current recommendations [15]. mRS scores collected at hospital discharge were also converted into health utility values, based on the UK EQ-5D-3L tariff, using a published mapping algorithm [16]. A QALY profile was generated for each trial participant using the area-under-the-curve method, assuming a baseline health utility value of zero [17] and linear interpolation between utility measurements at hospital discharge and at 3 and 6 months assessment points, accounting for survival [18]. We used 3- or 6-month mRS-derived health utilities in place of EQ-5D-5L-derived values if the latter were missing.
Analytical methods
Analyses of clinical outcomes, resource use and costs
Survival outcomes were analysed through the use of fixed-effect regression models with adjustment for age, sex, interval between the emergency call and the ambulance arrival at the scene, interval between the ambulance arrival and the administration of the study drug, cause of cardiac arrest, initial cardiac rhythm, whether the cardiac arrest was witnessed and whether CPR was performed by a bystander [9]. Between treatment-group differences in mean resource use and mean costs were estimated using two-sample Student’s t tests. Estimates of 95% confidence intervals (CIs) surrounding between-group differences in mean costs were obtained using nonparametric bootstrapping with replacement, based on 1000 replications [19].
Handling missing data
Multiple imputations using chained equations with predictive mean matching [20] was used to predict values for missing observations, assuming data were missing at random. Missing costs and health utility values were imputed at the level of resource category, health-related quality of life measure and assessment point, stratified by survival status at hospital discharge and treatment allocation in accordance with current recommendations [21]. Twenty imputed datasets were generated and used to inform the base-case and subsequent sensitivity and subgroup analyses. Parameter estimates were pooled across the 20 imputed datasets using Rubin’s rule to account for between and within-imputation components of variance terms associated with parameter estimates.
Within-trial economic evaluation
Bivariate linear regressions that take into account the correlation between each patient’s costs and effects were used to model total costs and total QALYs over the 6-month follow-up period. By specifying the treatment group as an indicator within each equation, the incremental costs and QALYs attributable to adrenaline were estimated, while controlling for baseline covariates (age, sex, time to first dose administration, cause of cardiac arrest, whether the cardiac arrest was witnessed, whether a bystander performed CPR and initial cardiac rhythm). Cost-effectiveness was expressed in terms of an incremental cost-effectiveness ratio (ICER), defined as the incremental adjusted cost of adrenaline divided by the incremental adjusted QALYs of adrenaline. The ICER was then compared with a range of cost-effectiveness threshold values for an additional QALY. ICERs falling below or in the region of £20,000 (€23,000) to £30,000 (€34,500) per QALY gained would, in general, be considered as representing a cost-effective use of UK NHS resources [11, 22]. Confidence intervals surrounding mean ICER values were not calculated as they are problematic to interpret if the ICER denominator is estimated close to zero and the interval extends to cover negative values [23]. This is because a negative ICER might equally imply lower costs and more effective treatment or greater costs and less effective treatment compared with placebo. There is no way of differentiating between these two qualitatively different scenarios from a confidence interval alone. Thus, we followed standard practice in economic evaluations to present mean ICER values without accompanying confidence intervals or standard errors. Instead, uncertainty surrounding mean cost-effectiveness estimates were characterised through a Monte Carlo method involving simulating 1000 replicates of the ICER from a joint distribution of incremental costs and incremental QALYs [24]. By calculating net monetary benefits for each of these simulated ICER values at alternative cost-effectiveness thresholds varying from £0 to £100,000 (€115,000) per QALY gained, the probability of cost-effectiveness of adrenaline (defined as the proportion of positive net monetary benefits at a given threshold level) was calculated and plotted as a cost-effectiveness acceptability curve [25].
Sensitivity analyses
A series of sensitivity analyses, summarised in Additional file 3, assessed the impact of varying features of the within-trial economic evaluation on cost-effectiveness. These included extending the perspective of analysis to cover broader societal resource use and costs and extending the time horizon to 12 months after the cardiac arrest event. Further economic modelling (described in the sections that follow) explored how results altered when the time horizon was extended to cover the lifetimes of cardiac arrest survivors and the scope extended to include recipients of donated organs.
Sub-group analyses
A series of pre-specified subgroup analyses, summarised in Additional file 4, explored whether the cost-effectiveness estimates based on the within-trial data altered by age, gender, time to first dose administration, cause of cardiac arrest, whether the cardiac arrest was witnessed, whether bystander performed CPR or initial cardiac rhythm.
Extrapolations of cost-effectiveness
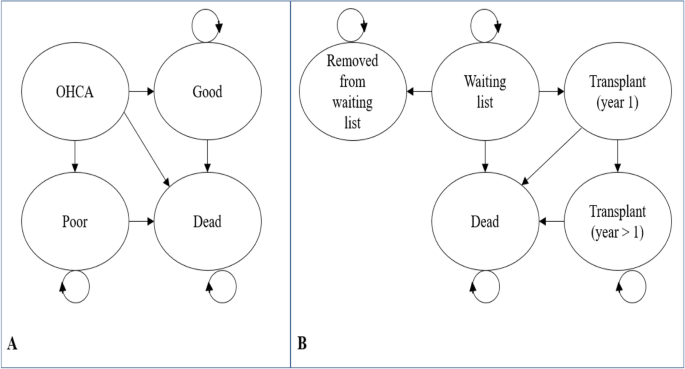
Controlled organ donation after neurological or cardiac death is available to families for patients who sustain un-survivable severe brain injury. Decision-analytic modelling was used to estimate the incremental cost-effectiveness of adrenaline beyond the parameters of the PARAMEDIC2 trial to include the costs and benefits of organ donation. A Markov state transition model was developed to extrapolate lifetime costs and benefits for out-of-hospital cardiac arrest survivors (Fig. 1, plot a). We separately adapted a previous economic model of adult lung transplantation [26] to simulate the impact on the cost-effectiveness of adrenaline resulting from changes in organ donation from deceased PARAMEDIC2 donors who had experienced out-of-hospital cardiac arrest. The model (Fig. 1, plot b) simulated pre and post-transplantation survival for individuals on the active national transplant waiting list. Epidemiological data for the model were informed by a separate linkage of the PARAMEDIC2 patients to the UK National Transplant Registry. Further details of the model structures, data inputs, analytical methods and description of methods for integrating the direct and indirect effects of adrenaline generated by the two models are provided in Additional file 5.
Model diagrams. Plot a: Model to extrapolate cost-effectiveness beyond PARAMEDIC2 trial follow-up. OHCA represents health state for out-of-hospital cardiac arrest patients. Good represents survival with good neurological function. Poor represents survival with poor neurological function. Plot b: Organ donor model adapted from Fisher et al. [26]
Results
A total of 8014 patients were randomised to either parenteral adrenaline (4015 patients) or saline placebo (3999 patients). In the adrenaline group, 130 patients (3.2%) were alive at 30 days, compared with 94 patients (2.4%) in the placebo group (unadjusted odds of survival, 1.39; 95% confidence interval [CI], 1.06 to 1.82; P = 0.02) [9]. Severe neurologic impairment at hospital discharge (mRS score of 4 or 5) was more common amongst survivors in the adrenaline group than in the placebo group (39 of 126 patients [31.0%] vs. 16 of 90 patients [17.8%]) [9].
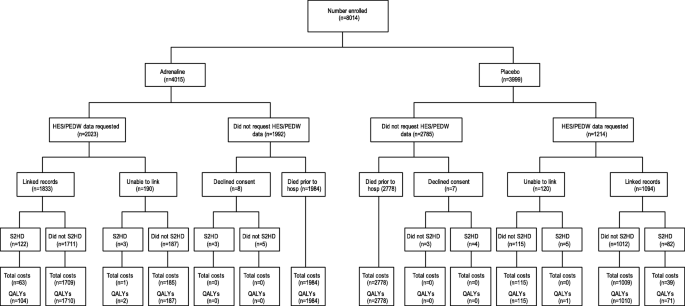
Figure 2 summarises the flow and completeness of data sources used to inform the economic parameters of the trial. Overall, between 1 and 2% of costs (at the component level) and approximately 1% of QALY data were missing (and subsequently imputed) for the primary analysis.
Resource use reported by the trial participants and or their proxies by treatment arm is summarised in Additional file 6. More patients were admitted to intensive care in the adrenaline arm (2016 vs 1209, P < 0.001), creating a larger group for assessment for donation after neurological or cardiac death. Linkage of the PARAMEDIC2 patients to the UK National Transplant Registry revealed that there were 99 recipients of organs donated by the adrenaline group compared with 67 recipients of organs donated by the placebo group (from 40 donors in the adrenaline group vs 24 donors in the placebo group). Breakdown by organ type is provided in Table 1.
Amongst all participants, mean total NHS and PSS costs over the 6-month trial time horizon was £3789 (€4357) in the adrenaline group vs. £2698 (€3103) in the placebo group [unadjusted mean cost difference £1091 (€1255) (bootstrap 95% CI £807 (€928) to £1398 (€1608)); P < 0.001] (Table 2). There was also a significant difference in mean total societal costs: £3829 (€4403) in the adrenaline group vs. £2687 (€3090) in the placebo group [unadjusted mean cost difference £1143 (€1314) (bootstrap 95% CI £861 (€990) to £1451 (€1669)); P < 0.001]. Amongst those who survived to hospital discharge, there were no significant differences in mean total NHS and PSS costs. However, amongst survivors to hospital discharge, mean utility values were significantly lower at hospital discharge in the adrenaline group [0.48 vs 0.60, unadjusted mean difference − 0.118 (95% CI − 0.196 to − 0.032); P = 0.002].
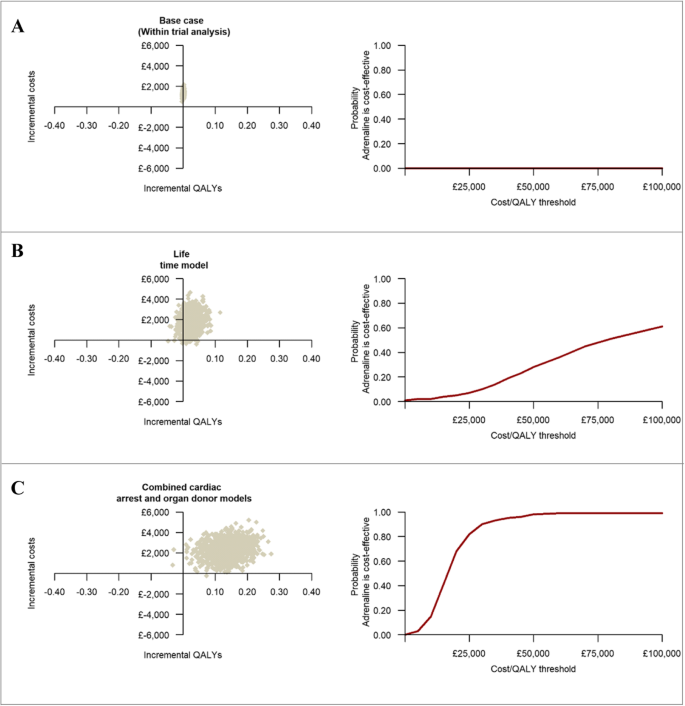
The within-trial economic evaluation results are summarised in Table 3. The base case analysis, over a 6-month time horizon, produced an ICER of £1,693,003 (€1,946,953) per QALY gained, reflecting significantly higher mean costs and only marginally higher mean QALYs in the adrenaline group. Extrapolating the within-trial analysis produced ICERs of £644,308 (€740,954) per QALY gained over 1 year and £81,070 (€93,231) per QALY gained over the lifetimes of survivors. The probability that adrenaline is cost-effective remained below 1% across a range of cost-effectiveness thresholds (plots a and b of Fig. 3), while mean net monetary benefits were negative at all thresholds. The cost-effectiveness estimates remained robust to a range of sensitivity and subgroup analyses (Additional file 7).
The three graphs on the left-hand side represent the cost-effectiveness plane for the within-trial, lifetime and the combined cardiac arrest and organ donor analyses. The three graphs on the right-hand side represent cost-effectiveness acceptability curves and give the probability that adrenaline is cost-effective compared with placebo at a specified cost-effectiveness threshold. Each cost-effectiveness plane is divided into four quadrants (North-West, North-East, South-West and South-East) by the intersection of the horizontal and vertical axis. South-East quadrant implies adrenaline is less costly and more effective than placebo, North-West quadrant implies adrenaline is less effective and more costly, the North-East quadrant implies adrenaline is more effective but also more costly and the South-West quadrant implies adrenaline is less effective but also less costly
The decision-analytic modelling that estimated combined direct economic effects over the lifetimes of survivors and indirect economic effects in organ recipients revealed that routine use of adrenaline at a UK-wide level in this patient group would result in incremental costs of £49,759,481 (€57,223,403) and 3093 additional QALYs per annum (Table 4). The mean ICER at a patient-level was estimated at £16,086 (€18,499) per QALY gained for adrenaline. The probability that adrenaline is cost-effective compared with placebo was 39% at a £15,000 (€17,250) per QALY cost-effectiveness threshold, increasing to 90% at a £30,000 (€34,500) threshold (Table 4 and plot c of Fig. 3). Sensitivity analyses revealed that these cost-effectiveness estimates remained robust to a range of modelling assumptions and data inputs with the exception of the estimated impact of the number of organs donated for transplantation on the cost-effectiveness of adrenaline (Additional file 5).
Discussion
This paper presents the first economic evaluation of adrenaline in adults with out-of-hospital cardiac arrest. The study revealed that when the assessment is restricted to the first 6 months that follow out-of-hospital cardiac arrest, adrenaline is associated with significantly higher mean costs, largely driven by the additional costs associated with hospital admissions, and only marginally higher mean QALYs. The base case analysis generated a mean ICER of £1,693,003 (€1,946,953) per QALY gained at 6 months post-randomisation, well in excess of accepted cost-effectiveness thresholds [11]. Consequently, the probability that adrenaline is cost-effective approximated to zero in the base-case analysis. Moreover, this conclusion remained robust after extensive sensitivity analyses that accounted for different sources of uncertainty. Notably, however, separate decision-analytic modelling that extrapolated cost-effectiveness over the lifetimes of survivors and incorporated additional costs and health consequences for organ recipients significantly altered the cost-effectiveness calculus. The probability that adrenaline is cost-effective increased to 90% at a £30,000 (€34,500) per QALY cost-effectiveness threshold, arguably within the bounds of acceptance by health technology agencies tasked with reimbursement decisions [11]. The cost-effectiveness conclusions were particularly sensitive to the inclusion of recipients of donated organs into economic modelling. The economic value of the additional survival and QALY benefits to organ recipients associated with adrenaline clearly outweigh the additional costs that result from organ transplantation. Similar findings have been reported for other interventions. In a study evaluating the costs and benefits of traumatic cardiopulmonary arrest resuscitation [27], the authors concluded that the financial burden associated with the procedure could be offset by expanding the benefits to include organ donation.
Previous economic evaluations of adrenaline were conducted in clinical contexts outside the management of out-of-hospital cardiac arrest [28,29,30], and therefore, a comparative assessment of cost-effectiveness evidence is not possible. Similarly, other pharmacological interventions for cardiac arrest, such as anti-arrythmics, have yet to be evaluated from an economic perspective. Our data should, therefore, be of relevance to clinical decision-makers and service planners tasked with the care and management of a condition that causes significant death and disability. Moreover, our economic evaluation is the first, to our knowledge, that has modelled the indirect economic benefits of organ donation arising from an intervention that increases the likelihood of admission to intensive care following an acute out-of-hospital clinical event. It may, therefore, alter methodological thinking on the incorporation of benefits other than directly to the patient into economic evaluations of interventions that affect the likelihood for organ donation. Such a methodological shift will require ethical consideration of how to balance benefits to others with benefits or harms to patients receiving the intervention.
Readers should consider caveats when interpreting the study results. First, our findings may not be applicable outside of the UK as the models of prehospital care, and healthcare costs may differ in other countries. Second, our adapted model of adult lung transplantation excluded the effects of adrenaline on pancreas and heart transplants. A paucity of epidemiological and economic evidence meant that we were unable to parameterise extensions to our decision-analytic model that considered effects beyond lung, liver and kidney transplants. Nevertheless, the number of pancreases and hearts transplanted to recipients of organs donated by the PARAMEDIC2 patients was small (< 10) and therefore unlikely to alter the balance of overall conclusions. Third, as with any health economic evaluation, there are likely to be potentially relevant effects that are not captured by the tools available for measurement and valuation. Specifically, we did not value other potential consequences of adrenaline, for example, the potential benefits to family members arising from the increased likelihood of being able to say goodbye and be present at the time of death [31]. Finally, in the modelling that extended our assessments of cost-effectiveness of adrenaline over the lifetimes of cardiac arrest survivors and incorporated the indirect economic effects of organ transplantation, a number of assumptions were required to simplify the parameterisation and address data limitations. Details of these assumptions are described in additional file 5. These included assuming that functional health state does not change over extended periods of survival and that resource use captured in the 3–6 month post-randomisation period can be extrapolated to cover the 6–12 month post-randomisation period. The organ donor modelling required a set of steady or equilibrium-state assumptions (number of organs available each year equals the number of transplantations with no wastage; the probability of death or removal on the waiting list are independent of organ supply) that captured the impact of intervention on the probability of transplantation. Nevertheless, our approach was consistent with current methodological guidance for decision-analytic modelling [32]. Furthermore, extensive sensitivity analyses were conducted that found that the conclusions from the base-case analyses were robust to doubling and halving input parameter values and alternative specifications of modelling assumptions.
Conclusion
In conclusion, adrenaline use in adults with out-of-hospital cardiac arrest does not represent a cost-effective use of resources when only directly related costs and consequences are considered. However, incorporating the indirect economic effects associated with transplanted organs substantially alters the cost-effectiveness calculus, suggesting decision-makers should consider the complexity of direct and indirect economic impacts of adrenaline.
Availability of data and materials
Not applicable
Abbreviations
- DBD:
-
Donation after brain death
- DCD:
-
Donation after circulatory death
- EVLP:
-
Ex vivo lung perfusion
- EVMP:
-
Ex vivo machine perfusion
- HES:
-
Hospital Episode Statistics
- HRG:
-
HealthCare Resource Group
- ICD:
-
Implantable cardioverter defibrillator
- ICER:
-
Incremental cost-effectiveness ratio
- NHS:
-
National Health Service
- NHS/PSS:
-
NHS and Personal Social Services
- NICE:
-
National Institute for Care Excellence
- PEDW:
-
Patient Episode Database for Wales
- QALY:
-
Quality-adjusted life year
- UK:
-
United Kingdom
- UKBT:
-
United Kingdom Blood and Transplant
References
Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation. 2010;81(11):1479–87.
Chan PS, McNally B, Tang F, Kellermann A, Group CS. Recent trends in survival from out-of-hospital cardiac arrest in the United States. Circulation. 2014;130(21):1876–82.
Perez CA, Samudra N, Aiyagari V. Cognitive and functional consequence of cardiac arrest. Curr Neurol Neurosci Rep. 2016;16(8):70.
Sandroni C, D'Arrigo S, Callaway CW, Cariou A, Dragancea I, Taccone FS, et al. The rate of brain death and organ donation in patients resuscitated from cardiac arrest: a systematic review and meta-analysis. Intensive Care Med. 2016;42(11):1661–71.
West S, Soar J, Callaway CW. The viability of transplanting organs from donors who underwent cardiopulmonary resuscitation: a systematic review. Resuscitation. 2016;108:27–33.
Snyder RA, Moore DR, Moore DE. More donors or more delayed graft function? A cost-effectiveness analysis of DCD kidney transplantation. Clin Transpl. 2013;27(2):289–96.
Dageforde LA, Feurer ID, Pinson CW, Moore DE. Is liver transplantation using organs donated after cardiac death cost-effective or does it decrease waitlist death by increasing recipient death? HPB (Oxford). 2013;15(3):182–9.
Perkins GD, Quinn T, Deakin CD, Nolan JP, Lall R, Slowther AM, et al. Pre-hospital assessment of the role of adrenaline: measuring the effectiveness of drug administration in cardiac arrest (PARAMEDIC-2): trial protocol. Resuscitation. 2016;108:75–81.
Perkins GD, Ji C, Deakin CD, Quinn T, Nolan JP, Scomparin C, et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med. 2018;379(8):711–21.
Haywood K, Whitehead L, Nadkarni VM, Achana F, Beesems S, Bottiger BW, et al. COSCA (Core outcome set for cardiac arrest) in adults: an advisory statement from the international liaison committee on resuscitation. Resuscitation. 2018;127:147–63.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. London, Department of Health. Available online from https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed 09 Sept 2020.
NHS Digital. HRG4+ 2016/17 Reference Costs Grouper 2017 London. 2017. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/grouper-and-tools-archive/costing-hrg4-2016-17-reference-costs-grouper.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36.
van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–15.
National Institute for Health and Care Excellence (NICE). Position statement on use of the EQ-5D-5L valuation set for England (updated November 2018). London: NICE; 2018. Report No.: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l.
Rivero-Arias O, Ouellet M, Gray A, Wolstenholme J, Rothwell PM, Luengo-Fernandez R. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Mak. 2010;30(3):341–54.
Dritsaki M, Achana F, Mason J, Petrou S. Methodological issues surrounding the use of baseline health-related quality of life data to inform trial-based economic evaluations of interventions within emergency and critical care settings: a systematic literature review. Pharmacoeconomics. 2017;35(5):501–15.
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press; 2005.
Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med. 2000;19(23):3219–36.
van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67.
Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics. 2014;32(12):1157–70.
Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1–503 v-vi.
Petrou S, Gray A. Economic evaluation using decision analytical modelling: design, conduct, analysis, and reporting. BMJ. 2011;342:d1766.
Glick HADJ, Sonnad SS, Polsky D. Economic evaluation in clinical trials. Oxford: Oxford University Press; 2014.
Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10(8):779–87.
Fisher A, Andreasson A, Chrysos A, Lally J, Mamasoula C, Exley C, et al. An observational study of donor ex vivo lung perfusion in UK lung transplantation: DEVELOP-UK. Health Technol Assess. 2016;20(85):1–276.
Love KM, Brown JB, Harbrecht BG, Muldoon SB, Miller KR, Benns MV, et al. Organ donation as an outcome of traumatic cardiopulmonary arrest: a cost evaluation. J Trauma Acute Care Surg. 2016;80(5):792–8.
Sumner A, Coyle D, Mitton C, Johnson DW, Patel H, Klassen TP, et al. Cost-effectiveness of epinephrine and dexamethasone in children with bronchiolitis. Pediatrics. 2010;126(4):623–31.
Shaker MS. An economic evaluation of prophylactic self-injectable epinephrine to prevent fatalities in children with mild venom anaphylaxis. Ann Allergy Asthma Immunol. 2007;99(5):424–8.
Fenwick E, Wilson J, Sculpher M, Claxton K. Pre-operative optimisation employing dopexamine or adrenaline for patients undergoing major elective surgery: a cost-effectiveness analysis. Intensive Care Med. 2002;28(5):599–608.
Kentish-Barnes N, Chaize M, Seegers V, Legriel S, Cariou A, Jaber S, et al. Complicated grief after death of a relative in the intensive care unit. Eur Respir J. 2015;45(5):1341–52.
Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices--overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Med Decis Mak. 2012;32(5):667–77.
Acknowledgements
The PARAMEDIC 2 trial team and the University of Warwick acknowledge the support of the National Institute for Health Research Clinical Research Network (NIHR CRN) and the Applied Research Centre (ARC) West Midlands. This project was funded by the National Institute for Health Research’s Health Technology Assessment Programme (project number 12/127/126).
We acknowledge NHS Blood and Transplant for access to data held on the UK Transplant Registry held by the Organ Donation and Transplantation Directorate and all those who have contributed data to the Registry.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Health Technology Assessment Programme, NIHR, NHS or the Department of Health.
PARAMEDIC 2 collaborators: Stavros Petrou, Jason Madan, Kamran Khan, Chen Ji, Anower Hossain, Ranjit Lall, Anne-Marie Slowther, Charles D. Deakin, Tom Quinn, Jerry P. Nolan, Helen Pocock, Nigel Rees, Michael Smyth, Simon Gates, Dale Gardiner, Gavin D. Perkins, Matthew Cooke, Sarah Lamb, Andrew Carson (RIP), Ian Jacobs (RIP), Ed England, John Black, Nicola Brock, Claire Godfrey, Sarah Taylor, Michelle Thomson, Isabel Rodriguez-Bachiller, Claire King, Marie Stevens, Johanna Lazarus, Helen Werts, Joshua Golding, Rachel Fothergill, Fionna Moore, Alex Boda, Richard Whitfield, Laura Galligan, Rob Lovett, Jennifer Bradley, Lyndsay O’Shea, Mark Docherty, Imogen Gunsen, Gill Price, Andy Rosser, Garry Parcell, Mindy Jhamat, Josh Miller, Jenny Sears Brown, Alice Pretty, Madison Larden, Emma Harris, Jenny Lumley-Holmes, Rhiannon Boldy, Prudence Horwood, Kyee Han, Karl Charlton, Sonia Byers, Gary Shaw, Matt Limmer, Craig Wynne, Michelle Jackson, Emma Bell, Oliver Gupta, Rima Gupta, Charlotte Scomparin, Susie Hennings, Jessica Horton, James Buck, Sarah Rumble, Hayley Johnson, Eva Kritzer, Chockalingham Muthiah, Adrian Willis, Claire Daffern, Louise Clarkson, Felix Achana, Nicola Cashin, Emma Skilton, Malvenia Richmond, Martin Underwood, Natalie Strickland, Sarah Duggan, Scott Regan, Marie Stevens, Jill Wood; Trial Steering Committee (Jon Nicholl, Neil Bayliss, Helen Snooks, Jonathan Benger, Robert Andrews, David Pitcher, William Lee, Matt Wise); Data Monitoring Committee (Marion Campbell, Jasmeet Soar, Kathy Rowan, Sue Mason). We would also like to thank collaborators at all receiving hospitals, all staff involved at participating ambulance services and our patient and public partners.
Funding
This project was funded by the National Institute for Health Research’s Health Technology Assessment Programme (project number 12/127/126).
Author information
Authors and Affiliations
Consortia
Contributions
FA, SP, KK, JM and DG contributed to the design of the within-trial cost-effectiveness analysis and the economic modelling. CJ, AH, RL and SG contributed to statistical design and analysed the data. AS, CD, TQ, JN, HP, NR, MS and GP contributed to the design of the study and data collection and provided clinical oversight. All authors contributed to drafting and proof-reading of the manuscript for high-quality intellectual work and that the findings and conclusions are consistent with the data. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The South Central–Oxford C Research Ethics Committee (REC: 14/SC/0157) and the Medicines and Healthcare Products Regulatory Authority (EudraCT: 2014-000792-11) approved the study protocol.
Consent for publication
Not applicable
Competing interests
MAS reports funding from the National Institute for Health Research. MAS has volunteer roles with the International Liaison Committee on Resuscitation, European and Resuscitation Council UK.
TQ reports grant funding paid to his institution by the National Institute for Health Research and British Heart Foundation. TQ has volunteer roles with the European Society of Cardiology.
GDP reports grant funding paid to his institution from the National Institute for Health Research, Resuscitation Council UK and British Heart Foundation related to this work. GDP has volunteer roles with the International Liaison Committee on Resuscitation, European and Resuscitation Council UK and Intensive Care Foundation. GDP is supported as a NIHR Senior Investigator and serves as an Editor for Resuscitation.
SP is supported by a Senior Investigator award from the UK National Institute of Health Research.
NR reports grants from National Institute for Health Research and Health and Care Research Wales during the conduct of the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
PARAMEDIC2 HEALTH ECONOMICS ANALYSIS PLAN. Details of the PARAMEDIC2 health economics analysis plan.
Additional file 2.
Unit costs for NHS, non-NHS and personal and social service resource inputs (£ sterling, 2017 prices). Unit costs data used to inform the within trial economic evaluation.
Additional file 3.
Pre-specified and post-hoc sensitivity analyses exploring varying features of the within-trial economic evaluation on the cost-effectiveness results. List of sensitivity analyses.
Additional file 4.
Subgroup analyses conducted that explored potential heterogeneity in the within-trial incremental cost-effectiveness of adrenaline.
Additional file 5.
Economic modelling to extrapolate cost-effectiveness beyond trial follow-up and incorporate the indirect effects of organ donation. List of subgroup analyses.
Additional file 6.
Resources use for NHS and personal and social services by trial arm in the 6 month post-randomisation period; all patients. Resource use results table, within-trial economic evaluation.
Additional file 7.
Cost-effectiveness (£, 2017 prices) of adrenaline based on within-trial economic evaluation; sensitivity analyses and subgroup analyses results. Additional cost-effectiveness results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Achana, F., Petrou, S., Madan, J. et al. Cost-effectiveness of adrenaline for out-of-hospital cardiac arrest. Crit Care 24, 579 (2020). https://doi.org/10.1186/s13054-020-03271-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-020-03271-0